This is the landing page for the dynorphin hypothesis of schizophrenia.
Schizophrenia appears to be a condition of extreme stress with issues in stress recovery, leading to an accumulation of negative consequences. There is evidence that extreme levels of stress may be hallucinogenic via Kappa Opioid Receptor (KOR) agonizing (1) and NMDAr blocking (2, 3) mechanisms. It may be that anyone can suffer psychosis with enough prolonged stress and suffering while those with schizophrenia are more susceptible to the effects of stress, less able to recover, and there may even be genes that predispose someone to seek out stressful and nonconformist situations naturally. In this section we will look at the hallucinogenic stress mechanisms.
Dynorphin seems to be capable of binding many theories of schizophrenia together. Dynorphins are a family of endogenous opioids that primarily bind to kappa opioid receptors (KORs) (4). This article will refer to this family of endogenous opioids as dynorphin. Dynorphin is involved in stress-induced dysphoria (5), pain-induced dysphoria (32, 33), anhedonia (6), anxiety (4, 7), addiction (8, 9), depressive (4), and even psychotic effects (10, 11, 12) . Dynorphin directly blocks NMDAr (2, 3) which would produce NMDAr hypoactivity, satisfying the glutamate hypothesis of schizophrenia (13). Dynorphin also reduces glutamate release (88). Chronically elevated dopamine is thought to play a role in schizophrenia (14) and upregulates dynorphin (15), likely through interactions at D1 and NMDAr complexes (16). Dynorphin potentiates D2 receptors (17) and reduces dopamine release (18), satisfying the dopamine hypothesis of schizophrenia (14). Dynorphin is implicated in trauma (19), explaining the association of schizophrenia with trauma (20, 21, 22, 23, 24). Dynorphin mediates the effects of social defeat stress (25) and upregulates from early childhood social isolation (26), satisfying the social defeat theory of schizophrenia (30, 31). Social isolation has also been found to induce schizophrenia-like changes in animals (27, 28, 29).

The link of social defeat and schizophrenia may represent how crucial social support is for dealing with symptoms of stress, potentially from any cause. If you are persecuted, it is likely an even worse stressor because not only can you not get social support, but you have social stress and offense. The severity of symptoms in schizophrenia correlated with a lack of friends (34). Frequent interactions with friends was found to be crucial to recovery in schizophrenia, more than the self-reported quality of friendships (35), although this likely doesn’t mean that abusive friends are better than none. Social connection seems to benefit schizophrenia, even through internet forums (36). A survey found that many schizophrenics have no friends and they believe this is not a problem (37). In an experimental study, reducing loneliness was found to reduce paranoia while inducing loneliness increased paranoia (38). These findings extend to genetic correlations, as a genome-wide association study found coheritability of loneliness and schizophrenia (39). Those with schizophrenia often feel excluded due to stigma (40), so the shame of having the disorder might worsen loneliness, which could perpetuate the severity of the condition. Also to reiterate, early-life social isolation enhances the dynorphin system (26).
Experimentally induced social threat induced paranoia (41). It may be that social exclusion, otherness, loneliness are all very stressful and hindering to stress recovery. The picture of social defeat may often begin with interest in the taboo and social deviance, which is found to occur before any psychotic symptoms in schizophrenics (42). To find a strange belief, such as flat earth theory, might ostracize oneself due to the stigma of such devious beliefs. Then as stigma and social exclusion sets in, symptoms get worse, perpetuating further exclusion. There will be no pressure to conform to normal belief systems in the absence of a normal group to conform to.
Socializing may be key to treating our stresses in life and for those who lack a social support system, they may be unable to recover and cope. Lacking a social support system may be one of the worst possible stressors when living in a society of humans. This seems to be the case with solitary confinement, which produces many schizophrenic symptoms in prisoners, including hallucinations, ideas of reference, persecutory delusions, and more (43). It seems likely that psychotic effects could emerge in anyone who is in an extremely aversive and helpless environmental condition, such as solitary confinement or torture, but those who are born with some set of genes linked to schizophrenia are more sensitive to aversive and helpless scenarios, or even more likely to find themselves in aversive and helpless living situations. This is further supported by the association found between low socioeconomic status and schizophrenia (44, 45, 46). Those with low socioeconomic status will be more likely to experience harsher living conditions, stress, and helplessness.
One might wonder if solitary confinement in prison is at least a more predictable living situation than isolation in the outside world. Those living in solitary confinement would sit without obligations or tasks to complete, without the fears of one’s life collapsing since it already has. Living in isolated conditions in the real world may still come with the obligations of paying rent, dealing with potential social threats, many stressors, fears of homelessness and life collapse. The biology and role of dynorphin in stress is explored in later sections, which will reveal the possibility that severe unfixable stress may eventually produce psychotic effects, to which individuals may have differing genetically-determined thresholds and sensitivites to such effects.
Individuals with schizophrenia often struggle with cognitive deficits. A genome-wide association study found extensive overlap with gene loci associated with poor cognitive performance and schizophrenia (47). Low IQ also correlates with schizophrenia (48) and dynorphin may be able to help explain this. Microinjections of dynorphin into the hippocampus of rats produced spatial memory deficits (49). Individuals with schizophrenia also face spatial memory deficits (50). Dynorphin was found to be elevated in age-related cognitive decline, where removing dynorphin related genes attenuated cognitive decline and has been studied in Alzheimer’s (51, 52, 53). Dynorphin has been associated to Down’s Syndrome (65). Alcohol-related memory and learning impairment is mediated by dynorphin upregulation (54). Stress-induced deficits in learning and memory are mediated by dynorphin (55). Occlusal disharmony, a painful condition, also involved memory and learning impairments mediated by dynorphin (56), which could be due to a role that dynorphin may play in pain aversion (32, 33).
Speaking of pain, dynorphin plays a role in PTSD (19) which is also linked to low IQ (57, 58, 59). Some research on PTSD and IQ has noted that premorbid IQ being lower also made PTSD worse (60), but this may be due to early life stress or that prior trauma is limiting IQ and even promoting more severe stress reactions in adulthood. For example, it was found that exposure to domestic violence suppressed IQ (61). Prior trauma is a risk factor for experiencing further trauma (62). So it seems likely that prior trauma and stress may be at play here with premorbid IQ scores. There is research showing up to a 14 point IQ difference under conditions of stress (63). Another genome-wide association study found strong evidence of genetic overlap between PTSD and schizophrenia (24). This supports the idea that individuals with schizophrenia are born with a higher sensitivity to stress.
On top of all of that, social exclusion was found to drastically decrease IQ (25%) and reasoning ability (30%) in the short term (64). This is important as social defeat stress is mediated by dynorphin. The low IQs found in schizophrenics might be explained by chronic rejection for being socially deviant.
Psychosis and dynorphin have been explored very lightly in the research so far. Kappa Opioid Receptor (KOR) agonists like dynorphin produce psychosis in healthy humans (1, 12, 66, 67, 68). It is thought that dynorphin releases during seizures to curb overactive glutamate activity, but also induces a psychosis post-seizure (69). Receptor complexes of dopamine D1 and D2 localize on dynorphin neurons in schizophrenia and meth users (70), which is important due to D1 receptor’s involvement in increased dynorphin activity (16). Dynorphin levels were found to be increased in the cerebral spinal fluid of schizophrenics and correlated with their psychotic symptom severity (10). Stimulants like cocaine (71), amphetamine (71), and nicotine (72) upregulate dynorphin activity which could explain related psychotic symptoms such as stimulant psychosis. There is something known as ‘coke bugs‘ which is formication, an effect that dynorphin injections were found to produce in 1 out of 4 subjects (73). Blocking dynorphin has also been shown to function as a rapid-acting antipsychotic (86, 87).
Exogenous KOR agonists have shown psychosis inducing effects. A quote from this study (1),
The inhalation of vaporized salvinorin-A led to very strong psychotropic effects of rapid onset and short duration. Perceptual modifications included the visual domain, and in contrast with 5HT2A agonists, auditory hallucinations were very common. Also in contrast with the classical serotonergic psychedelics, loss of contact with external reality was prominent with the participants being unreactive to external visual and verbal cues, especially after the medium and high doses. While at the low and medium doses there was an increase in bodily sensations, at 1.0mg there was an almost complete loss of body ownership and an increase in out-of-body experiences. These results suggest that the dynorphins – KOR system may play a previously underestimated role in the regulation of sensory perception, interoception, and the sense of body ownership in humans.
This study is great because it distinguishes the dynorphin-like psychotic effects such as hearing voices from serotonergic psychedelic states, which don’t often contain auditory voice hallucinations.
To recap, dynorphin is a KOR agonist similar to the drug Salvia Divinorum. It is essentially the body’s endogenous Salvia. This means social defeat and even stress, in general, should promote salvia-like hallucinogenic and psychotic effects. Those with genes related to schizophrenia likely have a natural sensitivity to stress, a decreased ability to recover from stress, a higher likelihood of living stressful lifestyles or engaging in potentially socially defeating behaviors.
Neurogenesis and Plasticity
Neurogenesis is the process of neuron birth. Neuroplasticity is the ability to reorganize synaptic connections, especially in response to learning. Neurogenesis in adults takes place primarily in the hippocampus and dentate gyrus (74, 75, 76). Individuals with schizophrenia have impaired neurogenesis (77) and neuroplasticity (79). One group of researchers tries to justify that schizophrenia could involve enhanced neurogenesis, probably because of the way NMDAr antagonists seem to induce neurogenesis (78). I’ve explained how this is wrong in the article Chemical Exorcism.
Impaired neurogenesis and stress were found to differentially lead to reduced hippocampal volume (80). Hippocampal volume was found to be reduced in schizophrenic and schizoaffective individuals but not bipolar 1 individuals (81). Neurogenesis is enhanced by Long Term Potentiation (LTP) (82) which relies on NMDAr function (83). There is evidence that schizophrenics have impaired LTP (84), likely mediated by NMDAr hypofunction. It is thought that this hypofunction of NMDAr seen in schizophrenia may explain the reduced neurogenesis (77). The NMDA receptor also plays a critical role in plasticity (85), so hypofunction of these receptors could also explain the reduced plasticity seen in schizophrenia.
Stress was found to reduce neurogenesis which may involve dynorphin, since it is implicated in stress reactions. Blocking dynorphin may lead to stress resilience (9) and functions as a rapid-acting and pronounced antipsychotic (86, 87). Dynorphin reduces LTP and reduces excitatory signaling in the hippocampus (88) and also the dentate gyrus (89), at least partly explaining the stress-linked reduction of hippocampal volume. Chronic opiate use both upregulates dynorphin activity (90) and inhibits neurogenesis (91). It isn’t clear that dynorphin inhibits neurogenesis itself, but it seems likely due to the above research. Some studies mention a hypothesis that dynorphin increases neuroplasticity. Addiction may certainly involve dynorphin upregulation, neuroplasticity, learning and LTP, but it also involves so many different mechanisms and fluctuations due to repeated dosing and withdrawals.
It is thought that increasing neuroplasticity may have potential in treating schizophrenia (92). Theanine is an NMDAr partial agonist that was found to function as an antipsychotic (93) and also enhances neurogenesis (94).
Glycine Switch
This mechanism is less well understood but is likely a crucial part of understanding what conditions influence the hallucinogenic effects of dynorphin. NMDAr has a glycine subsite which is a necessary cofactor for NMDAr stimulation. Glutamate can bind NMDAr if glycine is occupying the subsite receptor.
Dynorphin binds not only to the main receptor site of NMDAr as an antagonist (2, 3), but also appears to bind on the glycine subsite of NMDAr (95). Normally binding at the glycine subsite allows glutamate binding at NMDAr. When extracellular levels of glycine are low, dynorphin seems to replace glycine’s function, potentiating NMDAr activity (96). This suggests that dynorphin’s NMDAr blocking effects occur when glycine subsites are occupied either by glycine or dynorphin. When glycine is low, much of the dynorphin may be replacing glycine’s function. It could be that very high levels of dynorphin leads to the occupation of both the glycine subsite and the NMDAr main site by dynorphin, while when glycine levels are high, dynorphin occupies mostly the NMDAr site. If glycine levels are low, dynorphin probably functions as both NMDAr enhancer and antagonist, but primarily an enhancer at first.
Dynorphin may differentially produce dissociative or pain-sensitizing effects depending on extracellular glycine levels. If NMDAr activity is potentiated we should expect increased pain and excitotoxic effects (97). It may be that schizophrenics experience both of these depending on their glycinergic tone.
This becomes important in the KYNA section, but first we must understand the connection to:
Serotonin and Glutamate
Psychedelics may actually reveal a treatment for schizophrenia. This is against many people’s intuitions. The psychedelics are serotonergic agents. Some of the ones we will discuss here are LSD, psilocybin, DMT, and MDMA.
Psychedelic drugs induce neurogenesis in the hippocampus (98), a region that has reduced volume in individuals with schizophrenia (99). Serotonin is also able to induce neurogenesis (100). Psychedelics promote structural and functional neuroplasticity (101), which, as previously mentioned, is impaired in schizophrenia (79).
LSD was found to attenuate the depressive effects of dynorphin/KOR agonism (102). On the other hand, a 5HT2a inverse agonist (opposite of agonist) were found to potentiate KOR signaling (103). 5HT2a receptor agonism was found to inhibit aversion in the DPAG (104), a region of the brain in which KORs are located (105) and PTSD is implicated in (106). CBD was found to have antipsychotic properties (107) and is a 5HT2a and 5HT1a receptor agonist and thought to reduce aversion in the DPAG as well (108). Psychedelics have also recently been considered as treatment for PTSD (109).
The mechanism of LSD’s anti-dynorphin effects are likely to be 5HT2a and 5HT1a receptor mediated. 5HT1a receptors have been found to suppress the dynorphin release that occurs from applying a dopaminergic (127). 5HT2a receptors have been shown to enhance acetylcholine release in the hippocampus and prefrontal cortex (128) which is significant because one of acetylcholine’s targets, nAch alpha7, suppresses dynorphin release (129). The nAch alpha7 receptor is thought to be a mechanism that is impaired in schizophrenia, which has led to experimental treatments using nAch alpha7 receptor agonists (130). The 5HT2a receptor also suppresses mGlur2 activity (145), a glutamate receptor that may normally facilitate dynorphin activity (148). Both 5HT1a and 5HT2a have been linked to suppressing aversion (131, 104, 132, 108), which fits well since dynorphin is implicated in aversion and these serotonin mechanisms seem to suppress dynorphin.
Dynorphin has been implicated in depression (4). Meanwhile, a single large dose of psilocybin produced long-lasting (6 months+) strong decreases in depression in 80% of patients who were dying of terminal illness (110). In another study on psilocybin for depression, all patients showed benefits at 1 week after the dose, and many of them showed benefits many weeks later (111). The fMRI scans revealed that reduced blood flow to the amygdala induced by psilocybin correlated with reduced depressive symptoms. Dynorphin is known to control the gain on an amygdalar anxiety circuit (7), which further implies an interruption of dynorphin. A 2020 meta-analysis looked into the research on psilocybin for anxiety and depression and found reduced anxiety as well (115). On top of that, dynorphin plays a major role in addiction (9, 112) and psilocybin was able to get 80% of long-term smokers off of nicotine using only a single dose (113). A 2016 study found that psilocybin even reduces the pain of social exclusion (114) which has implications for the connection of dynorphin, social defeat, and schizophrenia. All of this together suggests that psychedelics may interact with dynorphin, reducing its’ mechanisms and effects.
Dynorphin and psychedelics relate to fear extinction. Fear extinction is impaired in schizophrenics (116). During fear extinction, KOR mRNA is found to be dramatically downregulated while fear conditioning shows a dramatic upregulation of KOR mRNA (117). Blocking dynorphin/KOR was found to block conditioned fear (117). DMT microdosing in mice was found to enhance fear extinction (118). Low doses of psilocybin were found to increase neurogenesis and enhance fear extinction. High doses appear to do the opposite (119). D-cycloserine was found to facilitate fear extinction and is studied for treatment of schizophrenia (120). There are studies on the psychedelic MDMA exploring fear extinction in relevance to PTSD (121), which involves altered fear extinction like schizophrenia (122). During fear recognition tasks that measure amygdala response, schizophrenics show hyperactivation of the amygdala to both fearful and even neutral faces (123) while with LSD users there is a reduced response of the amygdala (124).
This altered fear extinction brings us back to the idea that individuals with schizophrenia may be less capable of recovering from stressful events, leading to an accumulation of negative effects. It seems that serotonin and dynorphin function with opposing and interconnecting roles in resilience and stress. This interaction relationship between dynorphin and serotonin seems to occur at p38 MAPK, KOR signaling induces the serotonin transporter (SERT) to reuptake serotonin, producing a hypo-serotonergic state (125). This induction of SERT was necessary for dynorphin to produce aversion (126) which may be due to the reduction of serotonin’s anti-dynorphin/KOR effects.
Blocking SERT has been found to produce stress resilience (133). The removal of p38 MAPK on serotonergic neurons also produces stress resilience (134), likely by disrupting dynorphin. Blocking dynorphin directly leads to stress resilience as well (9). Serotonin itself is known to downregulate SERT (135). This means that increasing serotonin activity should downregulate SERT and reduce the ability of dynorphin to exert its’ aversive effects. Likewise, when dynorphin levels are high its’ induction of SERT will lower extracellular serotonin levels and prevent SERT from downregulating. This should be expected to perpetuate a stressed tone, until something else either decreases the stressful trigger or increase serotonin levels and disrupt the low serotonin tone. Repeated doses of the KOR agonist, Salvia, were also found to upregulate SERT (136). Schizophrenics also appear to have increased SERT levels (137). Social defeat stress in animal models has shown to upregulate SERT (138). Ultimately, a pattern of low serotonin activity and high dynorphin activity seems to be consistent. It may be that disruption of KOR mediated effects invoked by psychedelics stops this loop in which SERT is induced, thus allowing serotonin to accumulate again and restore a resilient state of mind.
Individuals with schizophrenia were found to have less functional 5HT2a receptors (139). A gene related to less 5HT2a receptors was linked to schizophrenia and subsequently the schizophrenic subjects tended to have lower 5HT2a receptor mRNA (140) (warning: this is a candidate gene study). A meta-analysis supported this trend, in a region-dependent way (East Asia had the opposite pattern, perhaps suggesting cultural or regionally evolved differences) (141). Schizophrenics also showed less 5HT2a receptor mRNA in the hippocampal formation (142). They also show reduced 5HT2 receptor binding in the prefrontal cortex (144, 183).
UPDATE: It’s important to note that the research on 5HT2a receptors and schizophrenia is mixed. One meta-analysis from 2006 found no association in post mortem (Li, Duan, & He 2006). Another one from 2014 found a moderate to large effect showing reduced 5HT2a receptor binding both post-mortem studies and with molecular imaging studies, even in unmedicated schizophrenics (Selvaraj, Arnone, Cappai, & Howes 2014). It may be that this association is more complex. It seems highly likely that people with endogenous psychedelic effects would be labeled as schizophrenic along with those experiencing more classic schizophrenic symptoms such as hearing voices. This presents a big problem, but let’s move on.
The 5HT2a receptor forms complexes with mGlur2, which is thought to be a key to the psychedelic effects (145). A study showed reduced function of these psychedelic receptor complexes in post-mortem brains of schizophrenic patients (143). Since mGlur2 agonism enhances 5HT2a binding (146), the reduced binding of 5HT2a may be explained by the reduced number of mGlur2 that are seen in post mortem schizophrenics (147). It’s worth noting, this study found increased 5HT2a receptors in post-mortem schizophrenics. This lower mGlur2 receptor density could mean the schizophrenics have a lower ability to activate the 5HT2a/psychedelic mechanism. 5HT2a receptor agonism required the mGlur2 receptor’s presence for its’ effects to be exterted in animals (162), so even though the effect of 5HT2a receptor is to reduce mGlur2 activity, an absent mGlur2 prevent effects from occuring.
In older studies this is backed up. Schizophrenics appear to be less sensitive to LSD (149) and also showed reduced effects from DMT, experiencing a lack of visual effects at doses that worked in non-schizophrenics (150).
Glycine-type drugs that can treat schizophrenia through NMDAr enhancement increase serotonin in the prefrontal cortex where 5HT2a receptors are (151). Another study found that low thalamocortical plasticity is modulated by dysfunctional 5HT2a receptors in schizophrenia (152). Recall from earlier, serotonin, glycine type drugs, and psychedelics induce plasticity.
A major symptom of schizophrenia is low cognitive function. Individuals with schizophrenia also show impaired associative learning (153). Psychedelics were found to be cognitive enhancing (154) and increase associative learning ability (155). 5HT2a receptor antagonists were able to reduce verbal memory and spatial memory in SSRI pretreated individuals (156). Taken together this could mean that the lower functioning of the 5HT2a receptors may allow un-attenuated dynorphin activity to disrupt cognitive function in schizophrenia, in similar ways that is seen with stress-related cognitive decline. Since psychedelics attenuate the effects of dynorphin/KOR, it might be that cognitive impairments related to dynorphin are also attenuated.
This would include aging, which I recently explored on a post about Alzheimer’s and Psychedelics. Curiously, the brains of schizophrenics were found to be 8 years older than their true age (157). Another study revealed mGlur2 loss to correlate with age, rather than schizophrenia diagnosis (158). 5HT2a receptor binding is also lost with age (159). Reduced binding of 5HT2a receptors is associated with cognitive impairment (228). Also, when dynorphin producing genes are removed from mice, age-related loss of mGlur1 was attenuated, along with age-related cognitive decline (160). Somehow dynorphin activity leads to the loss mGlur1 in aging, so perhaps dynorphin may be implicated in the loss of mGlur2 that is seen in aging as well. The loss of mGlur2 with aging would mean that 5HT2a binding decreases, allowing dynorphin’s effects to increase, in what seems to be a vicious cycle. This might provide a model for cognitive aging as a dynamic between serotonin, dynorphin, and glutamate. In the case of schizophrenia, it may explain some of the accelerated aging.
A lot of research has focused on the idea that psychedelics are an axiomatic model for schizophrenia. Recent studies on schizophrenia that utilized an mGlur2 agonist drug failed phase 3 trials (161). This drug worked as an agonist of mGlur2 which is one of the receptors involved in the 5HT2a effect (162). The relationship here is that 5HT2a binding decreases mGlur2 binding which allows for increased glutamate release that is normally inhibited by mGlur2. This experimental drug functioned on the mGlur2 receptor in the opposite direction as psychedelics, enhancing the receptor rather than decreasing activity. This drug was found to correct some NMDAr antagonist mediated deficits in gamma oscillations but not restore NMDAr antagonist mediated cognitive function deficits (163). It was found that mGlur2 agonists prevent the neurotoxicity induced by NMDAr antagonists (164), but there is evidence that psychedelics also prevent the neurotoxicity associated with the typical NMDAr antagonists that model psychosis (165). The drug may have failed due to potential dynorphin enhancing effects. Since mGlur2 agonism decreases glutamate release, it counteracts the glutamate enhancing effects of NMDAr antagonists (166). It is possible that the drug helps with certain symptoms by enhancing 5HT2a signaling, but also preventing some of the mechanisms of 5HT2a signaling by functioning in the opposite way.
Tolerance and receptor downregulation might be an issue. Short lasting psychedelics like DMT might produce less tolerance hypothetically, thus be preferred to longer lasting psychedelics like LSD. It was noticed that the later phase of LSD intoxication became more paranoid and seemingly psychotic which led to research show that chronic LSD use may be a model for psychosis (167). In this study, they mentioned changes to gene expression as an explanation. An alternate explanation could be that 5HT2a binding is reduced. Chronic administration of LSD was found to reduce 5HT2a binding in the brain (168) and produces tolerance to the effects (169), which is a pattern seen in schizophrenics, both reduced binding and less sensitivity to the psychedelic effects. It was found that both acute and chronic dosing of LSD produced increases of serotonin levels (170). This increased serotonin activity may be able to downregulate the serotonin transporter, thus reducing the ability of dynorphin to exert effects and allow a higher tendency towards increased serotonin tone in general. In a way, it may reverse the sensitivity to stress and enhance a tendency towards resilience. Chronic use of psychedelics might produce a withdrawal like state, leading to enhanced dynorphin signaling, which may worsen problems. Use of DMT infrequently might be able to avoid some of these problems due to its short duration. Frequent use might produce a schizophrenia-like long-term problem, much like how benzodiazepine use produces anxiety and seizure disorders with long-term use.
All of this together suggests psychedelics may be able to treat schizophrenia by disrupting psychotomimetic KOR signaling. It isn’t clear if frequent dosing would be required or, if like depression, a single dose might show lasting effects. I suspect that it will be a matter of stress exposure as the psychedelic system of mechanisms may help in resilience and adaptation. The next thing we should do is look for those diagnosed as schizophrenic who have had experience with psychedelics and do not use cannabis or hopefully any other drugs.
KYNA
In recent years, a novel kynurenic acid hypothesis of schizophrenia (171) emerged after it was discovered that schizophrenics have elevated kynurenic acid in their CSF (172). Like dynorphin, stress-induces a rises in KYNA (173). Kynurenic acid (KYNA) is a metabolite of L-tryptophan that blocks NMDAr, AMPAr, and Kainate glutamate receptors, and also blocks the glycine site of NMDAr (174). This has special implications for the dynorphin glycine switch because, by blocking the glycine site of NMDAr, it means glutamate cannot bind the NMDAr and glycine is not being utilized. This may displace other glycine molecules which then creates an environment of high extracellular glycine, in which dynorphin is more likely to act directly on NMDAr as an antagonist, rather than replacing glycine’s role in enhancing glutamate binding on the NMDAr. KYNAs other mechanisms are to block glutamate activity as well, functioning as another endogenous dissociative-like mechanism to stack with dynorphin’s dissociative hallucinogenic effects.
This also connects the strange association of niacin and schizophrenia, since niacin is a precursor to L-tryptophan. In some cases niacin has functioned as an effective treatment for schizophrenia (175). Psychosis is also a symptom of niacin deficiency (176). A study found that a lack of niacin flush is an indicator of schizophrenia (177, 178). A more recent 2016 study noted that this attenuated response to niacin was dependent on the phase of schizophrenic, as ultra high-risk for psychosis patients had an exaggerated flush response (179). Delusional parasitosis and formication related to pellagra has been effectively and rapidly treated by niacin in the past (180).
The niacin flush involves the release of serotonin from platelets, and was blocked by 85% using a specific 5HT2a receptor antagonist (181), which could mean that those with schizophrenia are not experiencing this serotonergic effect. Another study explored hot flushes and suggested that 5HT2a receptors seem to be a key role in hot flushes (182). Since schizophrenics have less binding at 5HT2a receptors this could possibly explain the reduced flush.
Since L-tryptophan is a precursor to serotonin synthesis, KYNA hyper-production may be a product of a failure to synthesize serotonin, or that serotonin synthesis is turned off because of frequent reuptake induced by dynorphin.
Why might KYNA synthesis increase?
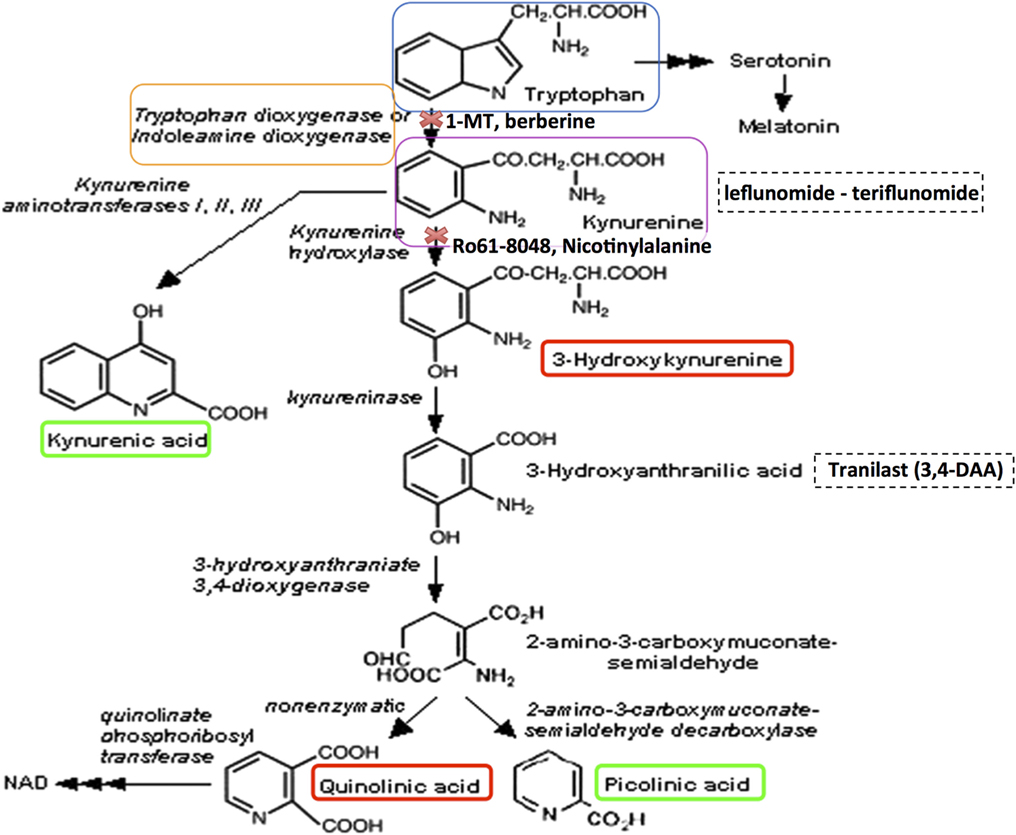
L-tryptophan has two pathways to metabolize, either the one leading to serotonin and melatonin, or the one leading to KYNA (184). Toxoplasmosis has been implicated in schizophrenia and was found to involve the secretion of an enzyme that leads to the degradation of tryptophan towards the KYNA pathway and away from the serotonin pathway, leading to a decrease in serotonin and increase in KYNA (185). Below is an example of the metabolic pathway taken from this paper (184). If it’s confusing, don’t worry just trekk on.
KYNA has also been linked to ketogenic diets, where increased levels of KYNA were shown to occur (186). This is significant because ketosis is considered a starvation state. There is evidence that ancestral famine leads to higher occurrence of schizophrenia in later generations (187). Ketosis is also associated with diabetes, which is associated to schizophrenia as well (188). Dynorphin levels are found to be increased in diabetes (189). Since serotonin suppresses hunger and regulates feeding (190) and dynorphin induces hunger (191), there may be an adaptation that occurs to prevent rising serotonin levels by interrupting the metabolic pathway of L-tryptophan to serotonin, instead favoring the KYNA pathway to dominate. This would keep one in a state of starvation and hunger, leading to seeking out extra food and surviving better than others during a famine. In this sense, some cases of schizophrenia may be a state similar to a perpetual starvation mindset.
This is further supported by the association of hibernation, feeding, and dynorphin (192). It could be that this starvation mechanism functions to induce hibernation eating patterns of storing food for the winter, which might be a natural recurring famine period in historic times. Protein consumption may be able to inhibit KYNA production (193), likely being one of the mediators that distinguishes the KYNA-serotonin pathway fork and functioning as a starvation switch. Individuals with schizophrenia also have higher BMI on average (194), which supports this famine hypothesis. Some of this ties into the recent post I did titled Junky Minds where you can read about how junk food may reduce our cognitive ability through dynorphinergic mechanisms.
KYNA also might antagonize nAch7 receptors (172) which has been shown to induce dynorphin release. The nAch7 receptors have been implicated in schizophrenia as well (130). The activation of these receptors is linked to a decrease in dynorphin release (129), suggesting that impairment of these receptors would disinhibit dynorphin release. Therefore KYNA may enhance dynorphin release by inhibiting nAch7 receptors.
KYNA may aide in binding the glycine switch and dynorphin parts of this hypothesis and also adds another stressor like sleep deprivation (195), may induce psychosis as well. Dietary, social, and/or sleep problems may begin one’s trend towards psychosis.
Dietary Influence
Relevant clips from Junky Mind:
A Plos One paper discusses the role that serotonin plays a role in insulin excretion from the pancreas (196). The authors note that diabetes occurs in the absence of serotonin. Because dynorphin has anti-serotonergic effects through inducing reuptake of serotonin, it seems likely that the rapid induction insulin-resistance due to pain (197) and stress (198) may be related to dynorphin, which is also implicated in both pain and stress aversion. In relation to schizophrenia, this could mean that pain and stress begin the loop of bad eating.
Dynorphin is further connected to dietary patterns through the orexin system, which regulates feeding behavior and energy balance (199). Dynorphin was found to induce feeding (191), putting a physiological touch to the notion of stress-eating. On the contrary, serotonin decreases hunger and is also implicated in energy balance (190). In regards to energy balance, this may be related to the role that dynorphin plays in depression (4), while serotonin seems capable of inducing mania (200), again, on the contrary. This fits within the hibernation and starvation KYNA hypothesis.
On the other hand, carbohydrate consumption is shown to mediate how much serotonin is released from neurons, how much serotonin synthesizes and induces a satiety effect (201). The paper notes that, unlike carbohydrates and proteins, fats are not associated with the production of any neurotransmitters, though this study was from 1995. Serotonin activation in the hypothalamus was found to reduce the consumption of fatty foods (202). Low fat and high carbohydrate diets have shown efficacy in reversing type II diabetes (203) (NutritionFacts). On the other hand, the high-fat ketogenic diet has been associated to insulin resistance, even while increasing energy expenditure and not leading to weight gain (204). Since ketogenic diets are low carb diets, they may limit serotonin synthesis and promote rising KYNA levels.
Despite this, ketogenic diets are being explored in treatment for schizophrenia, with seemingly good results. It may be that niacin deficiencies, low protein consumption, or junk food habits are resolved by the change in diet. There are cheese-derived and gluten-derived opioids that are thought to play a role in schizophrenia (205, 206). Many paleo-style ketogenic diets recommend against dairy or bread consumption, so this may be a factor as well. PsychologyToday noted that ketones may be providing energy to insulin-resistant brain cells. It is also possible the change to a low-carb diet halts the supply of energy to gut microbiota that are linked to schizophrenia (207).
One might consider how social factors play a role in junk food addiction. Social defeat stress was found to be mediated by dynorphin (25) and linked to weight gain and increased food intake (208). Like cocaine addiction and stress, social defeat upregulates the serotonin transporter (209), likely partly due to dynorphin. Socially subordinated monkeys had increased vulnerability to addiction to cocaine compared to dominant monkeys (210). In the article Serotonism, we explored how serotonin is implicated in social hierarchy of many species, revealing further contrary patterns in dynorphin and serotonin research. Low social rank is thought to be a risk factor for diabetes as well (211).
This further paints a picture of how general stress and bad experiences may accumulate and play together to manifest symptoms of schizophrenia. In this light, schizophrenia may be a low consciousness state that is focused on saving energy but also detecting potential threats, which paranoia may aide in. Major cities may be a hub for schizophrenia because of being such a place that requires constant higher consciousness while also being a very stressful place that slowly brings you into the depressive power-saver half-conscious state. Cities may also be a hub for junk food consumption among the poor.
The disorder may often begin to show up in the transition to adulthood because the individual loses their familial support such as money, resources, and general aide, while also gaining the freedom to stay up late at night, eat what they want, and live without rules to keep them in line. Many of the at-risk individuals may have already been living problematic lives with dwindling support from their families as they are given up on and left with less support. There are also toxic family dynamics that may occur, as I’ve described in Nexus.
Mutational Load and Social Defeat
The idea began as I read through Scott Alexander’s take on his blog SlateStarCodex in a post titled: Book Review: Evolutionary Psychopathology, which reported that schizophrenia and other mental health disorders are linked to higher mutational load. Mutational load is the decrease in fitness that accompanies high amounts of deleterious mutations. Mutations can lead to genetic diseases or benefits, but they tend to be negative more often than positive. This means that higher mutation rates should lead to increased mutational load. People with higher mutational load often have increased diversity of traits such as facial or other asymmetries. High mutational load is a social defeat risk factor, which is an element of one’s reproductive fitness as well. Those who have higher mutational load essentially have higher freakiness and lower relatability. For example, it is reported that facial asymmetry is related to higher mutational load and is also associated to schizophrenia (212, 213). The disorder is also associated to cerebral asymmetry as well (214). People like to relate to each other, it is validating, safe, comforting. Those with mutational load are essentially less compatible to the rest of society. Those who conflict with the world of consensus will be omitted from support systems of the society, thus more likely to be alone.
In Pandemic Xenophobia, we explored how kin selection promotes homogeneity, both genetic and probably also memetic similarity. Kin selection helps explain how self-sacrifice, or altruism, could evolve (215), despite altruism appearing to promote self-extinction while boosting the fitness of other organisms instead. The basic idea behind kin selection is that helping other organisms who share the same set of genes as you, can select for the genes that promote altruism. Next, since similarity to another provides the most benefits from altruism, this provides selection pressure for genetic similarity, or as W.D. Hamilton put it: relatability, which results in a slow progression to homogeneity. This is observed in hyper-altruistic species such as the naked mole rat, which turns out to be a highly inbred, almost clone-like species (216). Species like the naked mole rat or even ants have become altruistic enough that most of their colonies are comprised of individuals that do not even breed themselves.
There is evidence for a trend of a decreasing genetic diversity among humans throughout history (217). It may have been near extinction events or bottlenecks that initially drove a decrease of genetic diversity and as social benefits from social cohesion of this increasing genetic similarity emerge, the gene pool may continue to shrink due to the dynamics of kin selection that puts selection pressure on homogeneity.
Twins have been colloquially labeled as near-telepathic in their empathic bond with each other. While there may be no magic here, this tendency could be a product of how altruism maximizes in situations of homogeneity. This has been demonstrated in some research, exploring how monozygotic twins tend to be more aggressively altruistic with each other than dizygotic twins (218). One study noted that the gender mattered more than dizygosity (219), which suggests that it is mostly about perceived relatability, possibly indicating we only have a vague sense of relatability to others. It may strongly depend on low-conflict and cohesion among people, which may increase with genetic dissimilarity.
The trend for hyper-altruism to edge closer to twinness is observed in naked mole rats, who have a higher relatedness coefficient than siblings (0.5) coming in at 0.81 (216). This is quite incredible because they are coming closer to the relatedness coefficient of identical twins, which is 1.0. The implication of this is that naked mole rats within a colony are more related to each other than siblings generally are. Not only this, but they are even closer to being identical twins than they are to being siblings.
One problem though: the naked mole rats are extremely xenophobic (220). Their evolution towards homogeneity may have involved a tendency to push out those who were dissimilar (221). Perhaps they were mutated freaks, or Xenotypes. This isn’t the only reason to evolve xenophobia, but pandemics put pressure on keeping foreigners of a colony out because of the major risk for shared vulnerabilities to viruses or pathogens due to high relatedness. This is the idea explored in Pandemic Xenophobia, but for now we will move on.
This bias of our species to be altruistic towards those who we relate to essentially excludes those with higher mutational load. This higher mutational load is a kind of heterogeneity. They are the losing side of kin selection and they experience madness and suffering due to this. The social defeat theory is a story told by those who are denied access to the cultural system of altruism. Kin selection selects against mutational load by selecting for homogeneity. On the other hand, a constantly evolving and diverse environment might promote selection for increased mutation rate because those with higher mutation rates may be the ones who are more likely to have a beneficial mutation that survives the environment.
In Xenotypy, we explored a similar dynamic, with focuses on links of mental illness and Neanderthal associated DNA (222) (I am not so sure what I believe about this kind of research, it’s important to note). Neanderthals seemed to live in smaller group sizes, ultimately living less social lives. Some researchers have noted that autism-linked genes are associated to Neanderthal DNA and they are actively being selected out of our gene pool, meanwhile prosocial genes are being selected for (223).
Divergant thinking (DT) is associated to schizotypy and bipolarity positively but schizophrenia negatively (224). To explain this, it could make sense that schizotypy is the natural state whereas schizophrenia is post-traumatic sickness that results from a ruined schizotypal. In relation to schizophrenia, this fits with the conspiracy theory tendency of the schizophrenic. Radical ideas seem obviously divergent, but after being mocked and teased the schizophrenic phenotypes may lose their flexibility and plasticity to change their beliefs. As they identify with these radical belief systems and the baggage that comes with it (socially defeated identity), they may grow progressively sicker and have decreased DT.
DT can be viewed as an enhanced cognitive-cultural mutation, or more simply: memetic mutation rate. Divergant thoughts are cognitive mutations, which become memes for society. In the same way that genetic mutations promote evolution, so does memetic mutation. The dark side of this is that mutations can be good or bad, and so can thoughts. Those who are socially ruined because of their contribution to the meme-pool become schizophrenically sick, some of them at least. Others may become popular or maintain a manic tendency. For those who experience memetic success, their DT is rewarded and reinforced. For those who experience failure, their DT is punished and shamed, resulting in social trauma and extinction of their DT.
Those who are too different from popular phenotypes may be left at the curb-side of society’s altruism systems. This may result in psychiatric problems and a kind of ‘solitary confinement’ kind of lifestyle that produces schizophrenic symptoms.
Although this meme was originally about the idea of novelty-seeking driven sub-culture exploration that drives one to accumulate a rainbow pattern of cultural influences, this strangely applies to the concept of mutation load as well.

This is similar to the concept of discipline and cohesion in British politics, which is the notion that the Prime Minister is constantly kept in check by the threat of being ejected (discipline) and is driven to conform to the public’s desires (cohesion). There is an expectation for conformity and agreeability from social groups (cohesion) and those who do not conform to the public narratives will be socially punished or excluded from social interaction (discipline). Discpline and cohesion are the selection mechanism for the evolution memes. Those who are too different from popular phenotypes may be left at the curb-side of society’s altruism systems. This may result in psychiatric problems and a kind of ‘solitary confinement’ kind of lifestyle that produces schizophrenic symptoms.
Sidenote: This idea becomes more fascinating when you consider how politics really are memes. There is a culture war against flat earth theory and vaccines. Violent wars are driven by memetics as well.
Those who become traumatized or socially aversive may retreat away from humanity and invest their time learning alternative streams of information. This may be how autistic special interests develop, where the individual may neglect social development in favor of their special interest. This reduces the aversive social experience while replacing social reward with an alternative reward. This may also be how savantism occurs, as the childhood critical period may allow special abilities and skills to develop around the individual’s special interests. As the meme goes: start them early.
The schizophrenic may experience a similar social retreating but after the childhood critical period, perhaps making it even more difficult to recover, due to the lack of the critical period. It may not even necessarily relate to the timing of social retreat, but perhaps the schizophrenic more often involves a traumatic entry point to their social withdrawal. Since trauma has been shown to inhibit cognitive ability and decrease IQ, it could be that the special interests of the schizophrenic are more likely to be absurd, or even fear-centric, such as conspiracy theories. The idea of stress inhibiting developmental paths has been explored in Trauma Traps.
The social defeat model has been explored throughout this blog, especially in the essay Delusion, which argues that the concept of delusion is flawed, that it represents deviance from cultural beliefs and that there is no real ‘truth’ that we have attained about the outside world. There are indeed better or worse ideas, which education may shape, but to diagnose an uneducated and silly idea as a mental health problem seems problematic. Of course, a lack of education isn’t the only reason someone might have deviations from culture and most people do not actively deviate or create novel interpretations of the world, instead most submit to a dogmatic perspective on the data, for which society cohesively submits to. Divergent thinking may initially guide one away from popular narratives and into deviant territory that leads to social disciplinary action, rejection, and isolation for the individual.
From Delusion:
Those who believe in such unpopular opinions as flat-earth theory will be more at-risk for becoming alone. Those who are alone will struggle to assimilate to a culture in which they have restricted access to. There is simply nothing to assimilate to in their world, it is socially and culturally deprived. This would promote further deviation in the individual’s information pool because they are not acquiring information from popular culture or peers, but probably continuing down the only path they’ve known yet, the one that led to their isolation in the first place. This leads one into a state of social defeat.
Wikipedia defines social defeat as such:
“
The social defeat model has been extended to include observations of human aggression, bullying, relational aggression, chronic subordination and humiliation.
“
At this point, it’s worth noting that there is a social defeat hypothesis of schizophrenia. It was posited after noticing that stigmatized and less fortunate demographics had higher rates of schizophrenia. Clearly, transgenders are a stigmatized group which fits into this hypothesis well, also aiding in making sense of their immunity to illusions.
Furthermore, dynorphin mediates the effects of social defeat stress and upregulates from early childhood social isolation, both satisfying the social defeat theory of schizophrenia. The link between social defeat and schizophrenia may represent how crucial social support is for dealing with symptoms of stress, potentially from any cause. If you are widely persecuted, it is likely an even worse stressor than many others because not only can you not get social support, but you have social threat and offense.
Final Thoughts
Correcting the problems of schizophrenia may be complex and involve or require many different solutions. Solving the situational problems such as diet, poverty, social isolation, and abuse may prove difficult but fruitful in healing from psychotic problems. Psychedelics may facilitate a change of thinking, temporarily compromise trauma-response patterns, at least until their lifestyle caves in on them again. If 5HT2ar downregulates from psychedelic use, the person might hypothetically become more sensitive to these environmental problems, so this strategy may be best after life quality has improved. This warrants caution with the use of psychedelics because downregulation and desensitization could result in an increased propensity to react to a stressful environment with psychotic symptoms. Thus there may be a limited duration window of beneficial use with repeated psychedelic therapies.
The reality is that life quality is likely mediated by psychotic symptoms and their interaction with the social environment, due to shame, stigma, paranoia, and social distrust, so the solution may be a complex combination of freeing the person temporarily so that they can find ways to escape their problematic lifestyle situation and slowly improve their surroundings. Teaching critical thinking skills and educating the person where there are important gaps in knowledge would be important to aide in correcting problematic interpretations of reality based on misinformation. This may be very difficult to pull off but perhaps an environment designed for guiding people back, such as a month-long retreat may help to rehabilitate the psychotic persons. This may lead to long-lasting benefits in the long-run which is potentially superior to the traditional strategy of symptom suppression which involves drugs that produce undesirable symptoms such as withdrawals that may only worsen symptoms later on.
There has been exploration of using psychedelics to treat both homophobia (225) and homosexuality (226) (of course homosexuality isn’t an illness), which, while may sound humorous, at least shows that a kind of mindscape conversion therapy may be possible with psychedelic drugs. A reprogramming of delusional fixations may aide in social recovery, leading a person to consume more cultural information and grow onto the shared thoughts of their surrounding people, as opposed to isolating with their drifting mind into strange ideas. This flexibility effect of the psychedelic drug seems like it may be a re-opening of the childhood critical period (227), a plastic mindset that is geared towards radical changes of thinking. The child must undergo radical change, otherwise they might never move on from object impermanence to object permanence. Such shifts of perspective seem to be psychedelic.
The Phoenix Effect explores the hypothesis that psychedelics bring back childhood, so check that out if you are curious!
I’d like to thank Mr. Nobody (username) from the discord server for helping to collect sources in our database and discussing some of these ideas.
Special thanks to the six patrons: Melissa Bradley, Morgan Catha, Niklas Kokkola, Abhishaike Mahajan, Riley Fitzpatrick, and Charles Wright! Abhi is also the artist who created the cover image for Most Relevant. Please support him on instagram, he is an amazing artist! I’d also like to thank Annie Vu, Chris Byrd, and Kettner Griswold for your kindness and making these projects and the podcast possible through your donations.
If you’d like to support these projects like this, check out this page.
If you liked this, follow me on
You can also follow the discussion for this post on Reddit:
Updates

CITATIONS
- Maqueda, A. E., Valle, M., Addy, P. H., Antonijoan, R. M., Puntes, M., Coimbra, J., … & Barker, S. (2015). Salvinorin-A induces intense dissociative effects, blocking external sensory perception and modulating interoception and sense of body ownership in humans. International Journal of Neuropsychopharmacology, 18(12), pyv065.
- Chen, L., Gu, Y., & Huang, L. Y. (1995). The mechanism of action for the block of NMDA receptor channels by the opioid peptide dynorphin. Journal of Neuroscience, 15(6), 4602-4611.
- Chen, L., Gu, Y., & Huang, L. Y. (1995). The opioid peptide dynorphin directly blocks NMDA receptor channels in the rat. The Journal of physiology, 482(3), 575-581.
- Knoll, A. T., & Carlezon Jr, W. A. (2010). Dynorphin, stress, and depression. Brain research, 1314, 56-73.
- Land, B. B., Bruchas, M. R., Lemos, J. C., Xu, M., Melief, E. J., & Chavkin, C. (2008). The dysphoric component of stress is encoded by activation of the dynorphin κ-opioid system. Journal of Neuroscience, 28(2), 407-414.
- Muschamp, J. W., & Carlezon, W. A. (2013). Roles of nucleus accumbens CREB and dynorphin in dysregulation of motivation. Cold Spring Harbor perspectives in medicine, 3(2), a012005.
- Crowley, N. A., Bloodgood, D. W., Hardaway, J. A., Kendra, A. M., McCall, J. G., Al-Hasani, R., … & Lowell, B. B. (2016). Dynorphin controls the gain of an amygdalar anxiety circuit. Cell reports, 14(12), 2774-2783.
- Shippenberg, T. S., Zapata, A., & Chefer, V. I. (2007). Dynorphin and the pathophysiology of drug addiction. Pharmacology & therapeutics, 116(2), 306-321.
- Chavkin, C., & Koob, G. F. (2016). Dynorphin, dysphoria, and dependence: the stress of addiction. Neuropsychopharmacology, 41(1), 373.
- Heikkilä, L., Rimón, R., & Ternius, L. (1990). Dynorphin A and substance P in the cerebrospinal fluid of schizophrenic patients. Psychiatry research, 34(3), 229-236.
- Moustafa, S. R., Al-Rawi, K. F., Al-Dujaili, A. H., Supasitthumrong, T., Al-Hakeim, H. K., & Maes, M. (2020). The Endogenous Opioid System in Schizophrenia and Treatment Resistant Schizophrenia: Increased Plasma Endomorphin 2, and κ and μ Opioid Receptors are Associated with Interleukin-6.
- Clark, S. D., & Abi-Dargham, A. (2019). The role of dynorphin and the kappa opioid receptor in the symptomatology of schizophrenia: A review of the evidence. Biological psychiatry, 86(7), 502-511.
- Moghaddam, B., & Javitt, D. (2012). From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology, 37(1), 4-15.
- Howes, O. D., & Kapur, S. (2009). The dopamine hypothesis of schizophrenia: version III—the final common pathway. Schizophrenia bulletin, 35(3), 549-562.
- Cagniard, B., Balsam, P. D., Brunner, D., & Zhuang, X. (2006). Mice with chronically elevated dopamine exhibit enhanced motivation, but not learning, for a food reward. Neuropsychopharmacology, 31(7), 1362-1370.
- Hanson, G. R., Singh, N., Merchant, K., Johnson, M., & Gibb, J. W. (1995). The role of NMDA receptor systems in neuropeptide responses to stimulants of abuse. Drug and alcohol dependence, 37(2), 107-110.
- Escobar, A. P., González, M. P., Meza, R. C., Noches, V., Henny, P., Gysling, K., … & Andrés, M. E. (2017). Mechanisms of kappa opioid receptor potentiation of dopamine D2 receptor function in quinpirole-induced locomotor sensitization in rats. International Journal of Neuropsychopharmacology, 20(8), 660-669.
- Bruijnzeel, A. W. (2009). kappa-Opioid receptor signaling and brain reward function. Brain research reviews, 62(1), 127-146.
- Bailey, C. R., Cordell, E., Sobin, S. M., & Neumeister, A. (2013). Recent progress in understanding the pathophysiology of post-traumatic stress disorder. CNS drugs, 27(3), 221-232.
- Morgan, C., & Fisher, H. (2007). Environment and schizophrenia: environmental factors in schizophrenia: childhood trauma—a critical review. Schizophrenia bulletin, 33(1), 3-10.
- Sar, V., Taycan, O., Bolat, N., Özmen, M., Duran, A., Öztürk, E., & Ertem-Vehid, H. (2010). Childhood trauma and dissociation in schizophrenia. Psychopathology, 43(1), 33-40.
- Dennison, U., McKernan, D. P., Cryan, J. F., & Dinan, T. G. (2012). Schizophrenia patients with a history of childhood trauma have a pro-inflammatory phenotype. Psychological medicine, 42(9), 1865-1871.
- Lysaker, P. H., & LaRocco, V. A. (2008). The prevalence and correlates of trauma-related symptoms in schizophrenia spectrum disorder. Comprehensive Psychiatry, 49(4), 330-334.
- Duncan, L. E., Ratanatharathorn, A., Aiello, A. E., Almli, L. M., Amstadter, A. B., Ashley-Koch, A. E., … & Bradley, B. (2018). Largest GWAS of PTSD (N= 20 070) yields genetic overlap with schizophrenia and sex differences in heritability. Molecular psychiatry, 23(3), 666-673.
- Donahue, R. J., Landino, S. M., Golden, S. A., Carroll, F. I., Russo, S. J., & Carlezon Jr, W. A. (2015). Effects of acute and chronic social defeat stress are differentially mediated by the dynorphin/kappa-opioid receptor system. Behavioural pharmacology, 26(7 0 0), 654.
- Karkhanis, A. N., Rose, J. H., Weiner, J. L., & Jones, S. R. (2016). Early-life social isolation stress increases kappa opioid receptor responsiveness and downregulates the dopamine system. Neuropsychopharmacology, 41(9), 2263-2274.
- Li, B. J., Liu, P., Chu, Z., Shang, Y., Huan, M. X., Dang, Y. H., & Gao, C. G. (2017). Social isolation induces schizophrenia-like behavior potentially associated with HINT1, NMDA receptor 1, and dopamine receptor 2. Neuroreport, 28(8), 462.
- Jiang, Z., Rompala, G. R., Zhang, S., Cowell, R. M., & Nakazawa, K. (2013). Social isolation exacerbates schizophrenia-like phenotypes via oxidative stress in cortical interneurons. Biological psychiatry, 73(10), 1024-1034.
- Hardingham, G. E., & Do, K. Q. (2016). Linking early-life NMDAR hypofunction and oxidative stress in schizophrenia pathogenesis. Nature Reviews Neuroscience, 17(2), 125.
- Selten, J. P., van der Ven, E., Rutten, B. P., & Cantor-Graae, E. (2013). The social defeat hypothesis of schizophrenia: an update. Schizophrenia bulletin, 39(6), 1180-1186.
- Van der Ven, E. (2017). The social defeat hypothesis of schizophrenia: an update. European Psychiatry, 41(S1), S66-S66.
- Massaly, N., Copits, B. A., Wilson-Poe, A. R., Hipólito, L., Markovic, T., Yoon, H. J., … & Klaas, A. (2019). Pain-induced negative affect is mediated via recruitment of the nucleus accumbens kappa opioid system. Neuron, 102(3), 564-573.
- Cahill, C. M., Taylor, A. M., Cook, C., Ong, E., Morón, J. A., & Evans, C. J. (2014). Does the kappa opioid receptor system contribute to pain aversion?. Frontiers in pharmacology, 5, 253.
- Giacco, D., McCabe, R., Kallert, T., Hansson, L., Fiorillo, A., & Priebe, S. (2012). Friends and symptom dimensions in patients with psychosis: a pooled analysis. PLoS One, 7(11), e50119.
- Bjornestad, J., ten Velden Hegelstad, W., Joa, I., Davidson, L., Larsen, T. K., Melle, I., … & Bronnick, K. (2017). “With a little help from my friends” social predictors of clinical recovery in first-episode psychosis. Psychiatry Research, 255, 209-214.
- Haker, H., Lauber, C., & Rössler, W. (2005). Internet forums: a self‐help approach for individuals with schizophrenia?. Acta Psychiatrica Scandinavica, 112(6), 474-477.
- Harley, E. W. Y., Boardman, J., & Craig, T. (2012). Friendship in people with schizophrenia: a survey. Social psychiatry and psychiatric epidemiology, 47(8), 1291-1299.
- Lamster, F., Nittel, C., Rief, W., Mehl, S., & Lincoln, T. (2017). The impact of loneliness on paranoia: An experimental approach. Journal of behavior therapy and experimental psychiatry, 54, 51-57.
- Gao, J., Davis, L. K., Hart, A. B., Sanchez-Roige, S., Han, L., Cacioppo, J. T., & Palmer, A. A. (2017). Genome-wide association study of loneliness demonstrates a role for common variation. Neuropsychopharmacology, 42(4), 811-821.
- Killaspy, H., White, S., Lalvani, N., Berg, R., Thachil, A., Kallumpuram, S., … & Mezey, G. (2014). The impact of psychosis on social inclusion and associated factors. International Journal of Social Psychiatry, 60(2), 148-154.
- Saalfeld, V., Ramadan, Z., Bell, V., & Raihani, N. J. (2018). Experimentally induced social threat increases paranoid thinking. Royal Society open science, 5(8), 180569.
- Sobin, C., Blundell, M. L., Conry, A., Weiller, F., Gavigan, C., Haiman, C., & Karayiorgou, M. (2001). Early, non-psychotic deviant behavior in schizophrenia: a possible endophenotypic marker for genetic studies. Psychiatry research, 101(2), 101-113.
- Grassian, S., & Friedman, N. (1986). Effects of sensory deprivation in psychiatric seclusion and solitary confinement. International journal of law and psychiatry, 8(1), 49-65.
- Werner, S., Malaspina, D., & Rabinowitz, J. (2007). Socioeconomic status at birth is associated with risk of schizophrenia: population-based multilevel study. Schizophrenia bulletin, 33(6), 1373-1378.
- Link, B. G., Dohrenwend, B. P., & Skodol, A. E. (1987). Socioeconomic status and schizophrenia: Noisome occupational characteristics as a risk factor. In From Social Class to Social Stress (pp. 82-105). Springer, Berlin, Heidelberg.
- Agerbo, E., Sullivan, P. F., Vilhjalmsson, B. J., Pedersen, C. B., Mors, O., Børglum, A. D., … & Ripke, S. (2015). Polygenic risk score, parental socioeconomic status, family history of psychiatric disorders, and the risk for schizophrenia: a Danish population-based study and meta-analysis. JAMA psychiatry, 72(7), 635-641.
- Smeland, O. B., Bahrami, S., Frei, O., Shadrin, A., O’Connell, K., Savage, J., … & Ueland, T. (2020). Genome-wide analysis reveals extensive genetic overlap between schizophrenia, bipolar disorder, and intelligence. Molecular psychiatry, 25(4), 844-853.
- David, A. S., Malmberg, A., Brandt, L., Allebeck, P., & Lewis, G. (1997). IQ and risk for schizophrenia: a population-based cohort study. Psychological medicine, 27(6), 1311-1323.
- McDaniel, K. L., Mundy, W. R., & Tilson, H. A. (1990). Microinjection of dynorphin into the hippocampus impairs spatial learning in rats. Pharmacology Biochemistry and Behavior, 35(2), 429-435.
- Dreher, J. C., Banquet, J. P., Allilaire, J. F., Paillère-Martinot, M. L., Dubois, B., & Burnod, Y. (2001). Temporal order and spatial memory in schizophrenia: a parametric study. Schizophrenia Research, 51(2-3), 137-147.
- Jiang, H. K., Owyang, V. V., Hong, J. S., & Gallagher, M. (1989). Elevated dynorphin in the hippocampal formation of aged rats: relation to cognitive impairment on a spatial learning task. Proceedings of the National Academy of Sciences, 86(8), 2948-2951.
- Ménard, C., Tse, Y. C., Cavanagh, C., Chabot, J. G., Herzog, H., Schwarzer, C., … & Quirion, R. (2013). Knockdown of prodynorphin gene prevents cognitive decline, reduces anxiety, and rescues loss of group 1 metabotropic glutamate receptor function in aging. Journal of Neuroscience, 33(31), 12792-12804.
- Ménard, C., Herzog, H., Schwarzer, C., & Quirion, R. (2014). Possible role of dynorphins in Alzheimer’s disease and age-related cognitive deficits. Neurodegenerative Diseases, 13(2-3), 82-85.
- Kuzmin, A., Chefer, V., Bazov, I., Meis, J., Ögren, S. O., Shippenberg, T., & Bakalkin, G. (2013). Upregulated dynorphin opioid peptides mediate alcohol-induced learning and memory impairment. Translational psychiatry, 3(10), e310-e310.
- Carey, A. N., Lyons, A. M., Shay, C. F., Dunton, O., & McLaughlin, J. P. (2009). Endogenous κ opioid activation mediates stress-induced deficits in learning and memory. Journal of Neuroscience, 29(13), 4293-4300.
- Yamada, K., Ono, Y., Kubo, K. Y., Yamamoto, T., & Onozuka, M. (2013). Occlusal disharmony transiently impairs learning and memory in the mouse by increasing dynorphin A levels in the amygdala. The Tohoku Journal of Experimental Medicine, 230(1), 49-57.
- Kira, I., Lewandowski, L., Somers, C. L., Yoon, J. S., & Chiodo, L. (2012). The effects of trauma types, cumulative trauma, and PTSD on IQ in two highly traumatized adolescent groups. Psychological Trauma: Theory, Research, Practice, and Policy, 4(1), 128.
- Saltzman, K. M., Weems, C. F., & Carrion, V. G. (2006). IQ and posttraumatic stress symptoms in children exposed to interpersonal violence. Child Psychiatry and Human Development, 36(3), 261-272.
- Brandes, D., Ben-Schachar, G., Gilboa, A., Bonne, O., Freedman, S., & Shalev, A. Y. (2002). PTSD symptoms and cognitive performance in recent trauma survivors. Psychiatry research, 110(3), 231-238.
- Murray, B. (2002). The higher the IQ, the less likelihood of PTSD, study suggests. Monitor on Psychology, 33(4), 12.
- Koenen, K. C., Moffitt, T. E., Caspi, A., Taylor, A., & Purcell, S. (2003). Domestic violence is associated with environmental suppression of IQ in young children. Development and psychopathology, 15(2), 297-311.
- Spinhoven, P., Penninx, B. W., van Hemert, A. M., de Rooij, M., & Elzinga, B. M. (2014). Comorbidity of PTSD in anxiety and depressive disorders: Prevalence and shared risk factors. Child abuse & neglect, 38(8), 1320-1330.
- Mullainathan, S. (2014). FREEING UP. Scientific American Mind.
- Baumeister, R. F., Twenge, J. M., & Nuss, C. K. (2002). Effects of social exclusion on cognitive processes: anticipated aloneness reduces intelligent thought. Journal of personality and social psychology, 83(4), 817.
- Risser, D., You, Z. B., Cairns, N., Herrera-Marschitz, M., Seidl, R., Schneider, C., … & Lubec, G. (1996). Endogenous opioids in frontal cortex of patients with Down syndrome. Neuroscience letters, 203(2), 111-114.
- Healy, D. J., Damask, S. P., & Meador-Woodruff, J. H. (1994). Dopaminergic regulation of cortical prodynorphin derived peptides. Biological Psychiatry, 35(9), 690.
- Pfeiffer, A., Brantl, V., Herz, A., & Emrich, H. M. (1986). Psychotomimesis mediated by kappa opiate receptors. Science, 233(4765), 774-776.
- White, K. L., & Roth, B. L. (2012). Psychotomimetic effects of kappa opioid receptor agonists. Biological psychiatry, 72(10), 797-798.
- Bortolato, M., & Solbrig, M. V. (2007). The price of seizure control: dynorphins in interictal and postictal psychosis. Psychiatry research, 151(1-2), 139-143.
- Perreault, M. L., Hasbi, A., Alijaniaram, M., Fan, T., Varghese, G., Fletcher, P. J., … & George, S. R. (2010). The dopamine D1-D2 receptor heteromer localizes in dynorphin/enkephalin neurons increased high affinity state following amphetamine and in schizophrenia. Journal of Biological Chemistry, 285(47), 36625-36634.
- Hyman, S. E. (1996). Addiction to cocaine and amphetamine. Neuron, 16(5), 901-904.
- Tejeda, H. A., Natividad, L. A., Orfila, J. E., Torres, O. V., & O’Dell, L. E. (2012). Dysregulation of kappa-opioid receptor systems by chronic nicotine modulate the nicotine withdrawal syndrome in an age-dependent manner. Psychopharmacology, 224(2), 289-301.
- Wen, H. L., & Ho, W. K. (1982). Suppression of withdrawal symptoms by dynorphin in heroin addicts. European journal of pharmacology, 82(3-4), 183-186.
- Gage, F. H. (2002). Neurogenesis in the adult brain. Journal of Neuroscience, 22(3), 612-613.
- Gross, C. G. (2000). Neurogenesis in the adult brain: death of a dogma. Nature Reviews Neuroscience, 1(1), 67-73.
- Imayoshi, I., Sakamoto, M., Ohtsuka, T., & Kageyama, R. (2009). Continuous neurogenesis in the adult brain. Development, growth & differentiation, 51(3), 379-386.
- Reif, A., Schmitt, A., Fritzen, S., & Lesch, K. P. (2007). Neurogenesis and schizophrenia: dividing neurons in a divided mind?. European archives of psychiatry and clinical neuroscience, 257(5), 290-299.
- Na, K. S., Jung, H. Y., & Kim, Y. K. (2014). The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 48, 277-286.
- Bhandari, A., Voineskos, D., Daskalakis, Z. J., Rajji, T. K., & Blumberger, D. M. (2016). A review of impaired neuroplasticity in schizophrenia investigated with non-invasive brain stimulation. Frontiers in psychiatry, 7, 45.
- Schoenfeld, T. J., McCausland, H. C., Morris, H. D., Padmanaban, V., & Cameron, H. A. (2017). Stress and loss of adult neurogenesis differentially reduce hippocampal volume. Biological psychiatry, 82(12), 914-923.
- Arnold, S. J., Ivleva, E. I., Gopal, T. A., Reddy, A. P., Jeon-Slaughter, H., Sacco, C. B., … & Poudyal, G. (2015). Hippocampal volume is reduced in schizophrenia and schizoaffective disorder but not in psychotic bipolar I disorder demonstrated by both manual tracing and automated parcellation (FreeSurfer). Schizophrenia bulletin, 41(1), 233-249.
- Takeuchi, H., Kameda, M., Yasuhara, T., Sasaki, T., Toyoshima, A., Morimoto, J., … & Kuwahara, K. (2018). Long-term potentiation enhances neuronal differentiation in the chronic hypoperfusion model of rats. Frontiers in Aging Neuroscience, 10, 29.
- Lüscher, C., & Malenka, R. C. (2012). NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD). Cold Spring Harbor perspectives in biology, 4(6), a005710.
- Frantseva, M. V., Fitzgerald, P. B., Chen, R., Möller, B., Daigle, M., & Daskalakis, Z. J. (2008). Evidence for impaired long-term potentiation in schizophrenia and its relationship to motor skill leaning. Cerebral Cortex, 18(5), 990-996.
- Hunt, D. L., & Castillo, P. E. (2012). Synaptic plasticity of NMDA receptors: mechanisms and functional implications. Current opinion in neurobiology, 22(3), 496-508.
- Schmauss, C., Yassouridis, A., & Emrich, H. M. (1987). Antipsychotic effect of buprenorphine in schizophrenia. The American journal of psychiatry.
- Clark, S. D., Van Snellenberg, J. X., Lawson, J. M., & Abi-Dargham, A. (2020). Opioid antagonists are associated with a reduction in the symptoms of schizophrenia: a meta-analysis of controlled trials. Neuropsychopharmacology, 1-12.
- Wagner, J. J., Terman, G. W., & Chavkin, C. (1993). Endogenous dynorphins inhibit excitatory neurotransmission and block LTP induction in the hippocampus. Nature, 363(6428), 451-454.
- Terman, G. W., Wagner, J. J., & Chavkin, C. (1994). Kappa opioids inhibit induction of long-term potentiation in the dentate gyrus of the guinea pig hippocampus. Journal of Neuroscience, 14(8), 4740-4747.
- Werme, M., Thorén, P., Olson, L., & Brené, S. (2000). Running and cocaine both upregulate dynorphin mRNA in medial caudate putamen. European Journal of Neuroscience, 12(8), 2967-2974.
- Eisch, A. J., Barrot, M., Schad, C. A., Self, D. W., & Nestler, E. J. (2000). Opiates inhibit neurogenesis in the adult rat hippocampus. Proceedings of the National Academy of Sciences, 97(13), 7579-7584.
- Green, M. F. (2009). New possibilities in cognition enhancement for schizophrenia.
- Wakabayashi, C., Numakawa, T., Ninomiya, M., Chiba, S., & Kunugi, H. (2012). Behavioral and molecular evidence for psychotropic effects in L-theanine. Psychopharmacology, 219(4), 1099-1109.
- Takeda, A., Sakamoto, K., Tamano, H., Fukura, K., Inui, N., Suh, S. W., … & Yokogoshi, H. (2011). Facilitated neurogenesis in the developing hippocampus after intake of theanine, an amino acid in tea leaves, and object recognition memory. Cellular and molecular neurobiology, 31(7), 1079-1088.
- Voorn, P., van de Witte, S. V., wan Li, K., & Jonker, A. J. (2007). Dynorphin displaces binding at the glycine site of the NMDA receptor in the rat striatum. Neuroscience letters, 415(1), 55-58.
- Zhang, L., Peoples, R. W., Oz, M., Harvey-White, J., Weight, F. F., & Brauneis, U. (1997). Potentiation of NMDA receptor-mediated responses by dynorphin at low extracellular glycine concentrations. Journal of neurophysiology, 78(2), 582-590.
- Lai, J., Ossipov, M. H., Vanderah, T. W., Malan, T. P., & Porreca, F. (2001). Neuropathic pain: the paradox of dynorphin. Molecular interventions, 1(3), 160.
- Catlow, B. J., Jalloh, A., & Sanchez-Ramos, J. (2016). Hippocampal neurogenesis: Effects of psychedelic drugs. In Neuropathology of drug addictions and substance misuse (pp. 821-831). Academic Press.
- Thoma, R. J., Monnig, M., Hanlon, F. M., Miller, G. A., Petropoulos, H., Mayer, A. R., … & Cañive, J. M. (2009). Hippocampus volume and episodic memory in schizophrenia. Journal of the International Neuropsychological Society: JINS, 15(2), 182.
- Banasr, M., Hery, M., Printemps, R., & Daszuta, A. (2004). Serotonin-induced increases in adult cell proliferation and neurogenesis are mediated through different and common 5-HT receptor subtypes in the dentate gyrus and the subventricular zone. Neuropsychopharmacology, 29(3), 450-460.
- Ly, C., Greb, A. C., Cameron, L. P., Wong, J. M., Barragan, E. V., Wilson, P. C., … & Duim, W. C. (2018).Psychedelics promote structural and functional neural plasticity. Cell reports, 23(11), 3170-3182.
- Sakloth, F., Leggett, E., Moerke, M. J., Townsend, E. A., Banks, M. L., & Negus, S. S. (2019). Effects of acute and repeated treatment with serotonin 5-HT2A receptor agonist hallucinogens on intracranial self-stimulation in rats. Experimental and clinical psychopharmacology, 27(3), 215.
- Schreiber, S., Rigai, T., Katz, Y., & Pick, C. G. (2002). The antinociceptive effect of mirtazapine in mice is mediated through serotonergic, noradrenergic and opioid mechanisms. Brain research bulletin, 58(6), 601-605.
- Nogueira, R. L., & Graeff, F. G. (1995). Role of 5-HT receptor subtypes in the modulation of dorsal periaqueductal gray generated aversion. Pharmacology Biochemistry and Behavior, 52(1), 1-6.
- Gutstein, H. B., Mansour, A., Watson, S. J., Akil, H., & Fields, H. L. (1998). Mu and kappa opioid receptors in periaqueductal gray and rostral ventromedial medulla. Neuroreport, 9(8), 1777-1781.
- Brandão, M. L., & Lovick, T. A. (2019). Role of the dorsal periaqueductal gray in posttraumatic stress disorder: mediation by dopamine and neurokinin. Translational psychiatry, 9(1), 1-9.
- Hudson, R., Renard, J., Norris, C., Rushlow, W. J., & Laviolette, S. R. (2019). Cannabidiol counteracts the psychotropic side-effects of δ-9-tetrahydrocannabinol in the ventral hippocampus through bidirectional control of erk1–2 phosphorylation. Journal of Neuroscience, 39(44), 8762-8777.
- de Paula Soares, V., Campos, A. C., de Bortoli, V. C., Zangrossi Jr, H., Guimarães, F. S., & Zuardi, A. W. (2010). Intra-dorsal periaqueductal gray administration of cannabidiol blocks panic-like response by activating 5-HT1A receptors. Behavioural brain research, 213(2), 225-229.
- Sessa, B. (2017). MDMA and PTSD treatment:“PTSD: from novel pathophysiology to innovative therapeutics”. Neuroscience letters, 649, 176-180.
- Griffiths, R. R., Johnson, M. W., Carducci, M. A., Umbricht, A., Richards, W. A., Richards, B. D., … & Klinedinst, M. A. (2016). Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. Journal of psychopharmacology, 30(12), 1181-1197.
- Carhart-Harris, R. L., Roseman, L., Bolstridge, M., Demetriou, L., Pannekoek, J. N., Wall, M. B., … & Leech, R. (2017). Psilocybin for treatment-resistant depression: fMRI-measured brain mechanisms. Scientific reports, 7(1), 1-11.
- Shippenberg, T. S., Zapata, A., & Chefer, V. I. (2007). Dynorphin and the pathophysiology of drug addiction. Pharmacology & therapeutics, 116(2), 306-321.
- Johnson, M. W., Garcia-Romeu, A., Cosimano, M. P., & Griffiths, R. R. (2014). Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. Journal of psychopharmacology, 28(11), 983-992.
- Preller, K. H., Pokorny, T., Hock, A., Kraehenmann, R., Stämpfli, P., Seifritz, E., … & Vollenweider, F. X. (2016). Effects of serotonin 2A/1A receptor stimulation on social exclusion processing. Proceedings of the National Academy of Sciences, 113(18), 5119-5124.
- Goldberg, S. B., Pace, B. T., Nicholas, C. R., Raison, C. L., & Hutson, P. R. (2020). The experimental effects of psilocybin on symptoms of anxiety and depression: A meta-analysis. Psychiatry Research, 284, 112749.
- Holt, D. J., Lebron-Milad, K., Milad, M. R., Rauch, S. L., Pitman, R. K., Orr, S. P., … & Goff, D. C. (2009). Extinction memory is impaired in schizophrenia. Biological psychiatry, 65(6), 455-463.
- Knoll, A. T., Muschamp, J. W., Sillivan, S. E., Ferguson, D., Dietz, D. M., Meloni, E. G., … & Carlezon Jr, W. A. (2011). Kappa opioid receptor signaling in the basolateral amygdala regulates conditioned fear and anxiety in rats. Biological psychiatry, 70(5), 425-433.
- Cameron, L. P., Benson, C. J., DeFelice, B. C., Fiehn, O., & Olson, D. E. (2019). Chronic, intermittent microdoses of the psychedelic N, N-Dimethyltryptamine (DMT) produce positive effects on mood and anxiety in rodents. ACS chemical neuroscience, 10(7), 3261-3270.
- Catlow, B. J., Song, S., Paredes, D. A., Kirstein, C. L., & Sanchez-Ramos, J. (2013). Effects of psilocybin on hippocampal neurogenesis and extinction of trace fear conditioning. Experimental brain research, 228(4), 481-491.
- Cain, C. K., McCue, M., Bello, I., Creedon, T., Tang, D. I., Laska, E., & Goff, D. C. (2014). d-Cycloserine augmentation of cognitive remediation in schizophrenia. Schizophrenia research, 153(1-3), 177-183.
- Feduccia, A. A., & Mithoefer, M. C. (2018). MDMA-assisted psychotherapy for PTSD: are memory reconsolidation and fear extinction underlying mechanisms?. Progress in neuro-psychopharmacology and biological psychiatry, 84, 221-228.
- Norrholm, S. D., Jovanovic, T., Olin, I. W., Sands, L. A., Bradley, B., & Ressler, K. J. (2011). Fear extinction in traumatized civilians with posttraumatic stress disorder: relation to symptom severity. Biological psychiatry, 69(6), 556-563.
- Hall, J., Whalley, H. C., McKirdy, J. W., Romaniuk, L., McGonigle, D., McIntosh, A. M., … & Sprengelmeyer, R. (2008). Overactivation of fear systems to neutral faces in schizophrenia. Biological psychiatry, 64(1), 70-73.
- Dolder, P. C., Schmid, Y., Müller, F., Borgwardt, S., & Liechti, M. E. (2016). LSD acutely impairs fear recognition and enhances emotional empathy and sociality. Neuropsychopharmacology, 41(11), 2638-2646.
- Lemos, J. C., Roth, C. A., Messinger, D. I., Gill, H. K., Phillips, P. E., & Chavkin, C. (2012). Repeated stress dysregulates κ-opioid receptor signaling in the dorsal raphe through a p38α MAPK-dependent mechanism. Journal of Neuroscience, 32(36), 12325-12336.
- Schindler, A. G., Messinger, D. I., Smith, J. S., Shankar, H., Gustin, R. M., Schattauer, S. S., … & Chavkin, C. (2012). Stress produces aversion and potentiates cocaine reward by releasing endogenous dynorphins in the ventral striatum to locally stimulate serotonin reuptake. Journal of Neuroscience, 32(49), 17582-17596.
- Tomiyama, M., Kimura, T., Maeda, T., Kannari, K., Matsunaga, M., & Baba, M. (2005). A serotonin 5-HT1A receptor agonist prevents behavioral sensitization to L-DOPA in a rodent model of Parkinson’s disease. Neuroscience research, 52(2), 185-194.
- Nair, S. G., & Gudelsky, G. A. (2004). Activation of 5‐HT2 receptors enhances the release of acetylcholine in the prefrontal cortex and hippocampus of the rat. Synapse, 53(4), 202-207.
- Ji, L., Chen, Y., Wei, H., Feng, H., Chang, R., Yu, D., … & Zhang, M. (2019). Activation of alpha7 acetylcholine receptors reduces neuropathic pain by decreasing dynorphin A release from microglia. Brain research, 1715, 57-65.
- Lieberman, J. A., Dunbar, G., Segreti, A. C., Girgis, R. R., Seoane, F., Beaver, J. S., … & Hosford, D. A. (2013). A randomized exploratory trial of an alpha-7 nicotinic receptor agonist (TC-5619) for cognitive enhancement in schizophrenia. Neuropsychopharmacology, 38(6), 968-975.
- Melo, L. L., & Brandão, M. L. (1995). Role of 5-HT1A and 5-HT2 receptors in the aversion induced by electrical stimulation of inferior colliculus. Pharmacology Biochemistry and Behavior, 51(2-3), 317-321.
- Kuypers, K. P., De La Torre, R., Farre, M., Pizarro, N., Xicota, L., & Ramaekers, J. G. (2018). MDMA-induced indifference to negative sounds is mediated by the 5-HT 2A receptor. Psychopharmacology, 235(2), 481-490.
- Corchs, F., Nutt, D. J., Hood, S., & Bernik, M. (2009). Serotonin and sensitivity to trauma-related exposure in selective serotonin reuptake inhibitors-recovered posttraumatic stress disorder. Biological Psychiatry, 66(1), 17-24.
- Corchs, F., Nutt, D. J., Hood, S., & Bernik, M. (2009). Serotonin and sensitivity to trauma-related exposure in selective serotonin reuptake inhibitors-recovered posttraumatic stress disorder. Biological Psychiatry, 66(1), 17-24.
- Jørgensen, T. N., Christensen, P. M., & Gether, U. (2014). Serotonin-induced down-regulation of cell surface serotonin transporter. Neurochemistry international, 73, 107-112.
- Ramamoorthy, S (2019). Serotonin transporter phosphorylation in the behavioral effects of salvinorin A. Grantome. UNPUBLISHED
- Varnäs, K. (2005). Distribution of serotonin receptors and transporters in the human brain: implications for psychosis. Institutionen för klinisk neurovetenskap/Department of Clinical Neuroscience.
- Zhang, J., Fan, Y., Li, Y., Zhu, H., Wang, L., & Zhu, M. Y. (2012). Chronic social defeat up‐regulates expression of the serotonin transporter in rat dorsal raphe nucleus and projection regions in a glucocorticoid‐dependent manner. Journal of neurochemistry, 123(6), 1054-1068.
- Hazelwood, L. A., & Sanders-Bush, E. (2004). His452Tyr polymorphism in the human 5-HT2A receptor destabilizes the signaling conformation. Molecular pharmacology, 66(5), 1293-1300.
- Polesskaya, O. O., & Sokolov, B. P. (2002). Differential expression of the “C” and “T” alleles of the 5‐HT2A receptor gene in the temporal cortex of normal individuals and schizophrenics. Journal of neuroscience research, 67(6), 812-822.
- Abdolmaleky, H. M., Faraone, S. V., Glatt, S. J., & Tsuang, M. T. (2004). Meta-analysis of association between the T102C polymorphism of the 5HT2a receptor gene and schizophrenia. Schizophrenia research, 67(1), 53-62.
- López-Figueroa, A. L., Norton, C. S., López-Figueroa, M. O., Armellini-Dodel, D., Burke, S., Akil, H., … & Watson, S. J. (2004). Serotonin 5-HT1A, 5-HT1B, and 5-HT2A receptor mRNA expression in subjects with major depression, bipolar disorder, and schizophrenia. Biological psychiatry, 55(3), 225-233.
- Moreno, J. L., Miranda-Azpiazu, P., García-Bea, A., Younkin, J., Cui, M., Kozlenkov, A., … & Ge, Y. (2016). Allosteric signaling through an mGlu2 and 5-HT2A heteromeric receptor complex and its potential contribution to schizophrenia. Science signaling, 9(410), ra5-ra5.
- Hashimoto, T., Kitamura, N., Kajimoto, Y., Shirai, Y., Shirakawa, O., Mita, T., … & Tanaka, C. (1993). Differential changes in serotonin 5-HT 1A and 5-HT 2 receptor binding in patients with chronic schizophrenia. Psychopharmacology, 112(1), S35-S39.
- dos Santos, R. G. (2014). Psychedelics, Glutamate, and Neuroimaging Studies. The Journal of the American Society of Anesthesiologists, 120(6), 1521-1522.
- Snyder, S. H. (2008). A complex in psychosis. Nature, 452(7183), 38-39.
- González-Maeso, J., Ang, R. L., Yuen, T., Chan, P., Weisstaub, N. V., López-Giménez, J. F., … & Gingrich, J. A. (2008). Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature, 452(7183), 93-97.
- Liu, N. J., Murugaiyan, V., Storman, E. M., Schnell, S. A., Kumar, A., Wessendorf, M. W., & Gintzler, A. R. (2017). Plasticity of signaling by spinal estrogen receptor α, κ-opioid receptor, and metabotropic glutamate receptors over the rat reproductive cycle regulates spinal endomorphin 2 antinociception: relevance of endogenous-biased agonism. Journal of Neuroscience, 37(46), 11181-11191.
- Liddell, D. W., & Weil-Malherbe, H. (1953). THE EFFECTS OF METHEDRINE AND OF LYSERGIC ACID DIETHYLAMIDE ON MENTAL PROCESSES AND ON THE BLOOD ANDRENALINE LEVEL. Journal of neurology, neurosurgery, and psychiatry, 16(1), 7.
- Böszörményi, Z. (1958). Dimethyltryptamine experiments with psychotics. Journal of mental science, 104(435), 445-453.
- Bannai, M., Kawai, N., Nagao, K., Nakano, S., Matsuzawa, D., & Shimizu, E. (2011). Oral administration of glycine increases extracellular serotonin but not dopamine in the prefrontal cortex of rats. Psychiatry and clinical neurosciences, 65(2), 142-149.
- Barre, A., Berthoux, C., De Bundel, D., Valjent, E., Bockaert, J., Marin, P., & Bécamel, C. (2016). Presynaptic serotonin 2A receptors modulate thalamocortical plasticity and associative learning. Proceedings of the National Academy of Sciences, 113(10), E1382-E1391.
- Diwadkar, V. A., Flaugher, B., Jones, T., Zalányi, L., Ujfalussy, B., Keshavan, M. S., & Erdi, P. (2008). Impaired associative learning in schizophrenia: behavioral and computational studies. Cognitive Neurodynamics, 2(3), 207.
- Zhang, G., & Stackman Jr, R. W. (2015). The role of serotonin 5-HT2A receptors in memory and cognition. Frontiers in pharmacology, 6, 225.
- Harvey, J. A. (2003). Role of the serotonin 5-HT2A receptor in learning. Learning & Memory, 10(5), 355-362.
- Wingen, M., Kuypers, K. P. C., & Ramaekers, J. G. (2007). Selective verbal and spatial memory impairment after 5-HT1A and 5-HT2A receptor blockade in healthy volunteers pre-treated with an SSRI. Journal of Psychopharmacology, 21(5), 477-485.
- Hajek, T., Franke, K., Kolenic, M., Capkova, J., Matejka, M., Propper, L., … & Kopecek, M. (2019). Brain age in early stages of bipolar disorders or schizophrenia. Schizophrenia bulletin, 45(1), 190-198.
- Frank, E., Newell, K. A., & Huang, X. F. (2011). Density of metabotropic glutamate receptors 2 and 3 (mGluR2/3) in the dorsolateral prefrontal cortex does not differ with schizophrenia diagnosis but decreases with age. Schizophrenia research, 128(1-3), 56-60.
- Meltzer, C. C., Smith, G., Price, J. C., Reynolds III, C. F., Mathis, C. A., Greer, P., … & Cantwell, M. N. (1998). Reduced binding of [18F] altanserin to serotonin type 2A receptors in aging: persistence of effect after partial volume correction. Brain Research, 813(1), 167-171.
- Ménard, C., Tse, Y. C., Cavanagh, C., Chabot, J. G., Herzog, H., Schwarzer, C., … & Quirion, R. (2013). Knockdown of prodynorphin gene prevents cognitive decline, reduces anxiety, and rescues loss of group 1 metabotropic glutamate receptor function in aging. Journal of Neuroscience, 33(31), 12792-12804.
- Li, M. L., Hu, X. Q., Li, F., & Gao, W. J. (2015). Perspectives on the mGluR2/3 agonists as a therapeutic target for schizophrenia: still promising or a dead end?. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 60, 66-76.
- Moreno, J. L., Holloway, T., Albizu, L., Sealfon, S. C., & González-Maeso, J. (2011). Metabotropic glutamate mGlu2 receptor is necessary for the pharmacological and behavioral effects induced by hallucinogenic 5-HT2A receptor agonists. Neuroscience letters, 493(3), 76-79.
- Sokolenko, E., Hudson, M. R., Nithianantharajah, J., & Jones, N. C. (2019). The mGluR2/3 agonist LY379268 reverses NMDA receptor antagonist effects on cortical gamma oscillations and phase coherence, but not working memory impairments, in mice. Journal of Psychopharmacology, 33(12), 1588-1599.
- Carter, K., Dickerson, J., Schoepp, D. D., Reilly, M., Herring, N., Williams, J., … & Sharp, F. R. (2004). The mGlu2/3 receptor agonist LY379268 injected into cortex or thalamus decreases neuronal injury in retrosplenial cortex produced by NMDA receptor antagonist MK-801: possible implications for psychosis. Neuropharmacology, 47(8), 1135-1145.
- Farber, N. B., Hanslick, J., Kirby, C., McWilliams, L., & Olney, J. W. (1998). Serotonergic agents that activate 5HT 2A receptors prevent NMDA antagonist neurotoxicity. Neuropsychopharmacology, 18(1), 57-62.
- Lorrain, D., Baccei, C. S., Bristow, L. J., Anderson, J. J., & Varney, M. A. (2003). Effects of ketamine and N-methyl-D-aspartate on glutamate and dopamine release in the rat prefrontal cortex: modulation by a group II selective metabotropic glutamate receptor agonist LY379268. Neuroscience, 117(3), 697-706.
- Marona-Lewicka, D., Nichols, C. D., & Nichols, D. E. (2011). An animal model of schizophrenia based on chronic LSD administration: old idea, new results. Neuropharmacology, 61(3), 503-512.
- Buckholtz, N. S., Freedman, D. X., & Middaugh, L. D. (1985). Daily LSD administration selectively decreases serotonin2 receptor binding in rat brain. European journal of pharmacology, 109(3), 421-425.
- Freedman, D. X., Appel, J. B., Hartman, F. R., & Molliver, M. E. (1964). Tolerance to behavioral effects of LSD-25 in rat. Journal of Pharmacology and Experimental Therapeutics, 143(3), 309-313.
- Lee, E. H. Y., & Geyer, M. A. (1980). Persistent effects of chronic administration of LSD on intracellular serotonin content in rat midbrain. Neuropharmacology, 19(10), 1005-1007.
- Erhardt, S., Schwieler, L., Nilsson, L., Linderholm, K., & Engberg, G. (2007). The kynurenic acid hypothesis of schizophrenia. Physiology & behavior, 92(1-2), 203-209.
- Erhardt, S., Blennow, K., Nordin, C., Skogh, E., Lindström, L. H., & Engberg, G. (2001). Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neuroscience letters, 313(1-2), 96-98.
- Chiappelli, J., Pocivavsek, A., Nugent, K. L., Notarangelo, F. M., Kochunov, P., Rowland, L. M., … & Hong, L. E. (2014). Stress-induced increase in kynurenic acid as a potential biomarker for patients with schizophrenia and distress intolerance. JAMA psychiatry, 71(7), 761-768.
- PubChem: Kynurenic Acid https://pubchem.ncbi.nlm.nih.gov/compound/kynurenic_acid
- Hoffer, A., Saul, A. W., & Foster, H. D. (2012). Niacin: The real story. Basic Health Publications.
- Hoffer, A. (1970). Pellagra and schizophrenia. Psychosomatics, 11(5), 522-525.
- Chang, S. S., Liu, C. M., Lin, S. H., Hwu, H. G., Hwang, T. J., Liu, S. K., … & Chen, W. J. (2009). Impaired flush response to niacin skin patch among schizophrenia patients and their nonpsychotic relatives: the effect of genetic loading. Schizophrenia bulletin, 35(1), 213-221.
- Smesny, S., Rosburg, T., Riemann, S., Baur, K., Rudolph, N., Berger, G., & Sauer, H. (2005). Impaired niacin sensitivity in acute first-episode but not in multi-episode schizophrenia. Prostaglandins, leukotrienes and essential fatty acids, 72(6), 393-402.
- Berger, G. E., Smesny, S., Schäfer, M. R., Milleit, B., Langbein, K., Hipler, U. C., … & Holzer, I. (2016). Niacin skin sensitivity is increased in adolescents at ultra-high risk for psychosis. PLoS One, 11(2), e0148429.
- Prakash, R., Gandotra, S., Singh, L. K., Das, B., & Lakra, A. (2008). Rapid resolution of delusional parasitosis in pellagra with niacin augmentation therapy. General hospital psychiatry, 30(6), 581-584.
- Papaliodis, D., Boucher, W., Kempuraj, D., Michaelian, M., Wolfberg, A., House, M., & Theoharides, T. C. (2008). Niacin-induced “flush” involves release of prostaglandin D2 from mast cells and serotonin from platelets: evidence from human cells in vitro and an animal model. Journal of Pharmacology and Experimental Therapeutics, 327(3), 665-672.
- Berendsen, H. H. (2000). The role of serotonin in hot flushes. Maturitas, 36(3), 155-164.
- Hashimoto, T., Kitamura, N., Kajimoto, Y., Shirai, Y., Shirakawa, O., Mita, T., … & Tanaka, C. (1993). Differential changes in serotonin 5-HT 1A and 5-HT 2 receptor binding in patients with chronic schizophrenia. Psychopharmacology, 112(1), S35-S39.
- Lovelace, M. D., Varney, B., Sundaram, G., Franco, N. F., Ng, M. L., Pai, S., … & Brew, B. J. (2016). Current evidence for a role of the kynurenine pathway of tryptophan metabolism in multiple sclerosis. Frontiers in immunology, 7, 246.
- BaharakKhabazghazvini. (2012).IDO1 (indoleamine 2,3-dioxygenase 1) has a role in the Psychiatric manifestations of Latent Toxoplasmosis? Metabolomics:Open Access, ISSN: 2153-0769
- Żarnowski, T., Chorągiewicz, T., Tulidowicz-Bielak, M., Thaler, S., Rejdak, R., Żarnowska, I., … & Gasior, M. (2012). Ketogenic diet increases concentrations of kynurenic acid in discrete brain structures of young and adult rats. Journal of Neural Transmission, 119(6), 679-684.
- Marris, E. (2005). Starving linked to schizophrenia. Nature.
- Guest, P. C. (2019). Insulin resistance in schizophrenia. In Reviews on Biomarker Studies of Metabolic and Metabolism-Related Disorders (pp. 1-16). Springer, Cham.
- Jolivalt, C. G., Jiang, Y., Freshwater, J. D., Bartoszyk, G. D., & Calcutt, N. A. (2006). Dynorphin A, kappa opioid receptors and the antinociceptive efficacy of asimadoline in streptozotocin-induced diabetic rats. Diabetologia, 49(11), 2775-2785.
- Donovan, M. H., & Tecott, L. H. (2013). Serotonin and the regulation of mammalian energy balance. Frontiers in neuroscience, 7, 36.
- Morley, J. E., & Levine, A. S. (1985). Dynorphin, an endogenous stimulator of feeding. Progress in clinical and biological research, 192, 293-300.
- Kretchmer, N. (1990). Childhood obesity: a biobehavioral perspective. CRC Press.
- Sekine, A., Okamoto, M., Kanatani, Y., Sano, M., Shibata, K., & Fukuwatari, T. (2015). Amino acids inhibit kynurenic acid formation via suppression of kynurenine uptake or kynurenic acid synthesis in rat brain in vitro. SpringerPlus, 4(1), 1-10.
- Coodin, S. (2001). Body mass index in persons with schizophrenia. The Canadian Journal of Psychiatry, 46(6), 549-555.
- Waters, F., Chiu, V., Atkinson, A., & Blom, J. D. (2018). Severe sleep deprivation causes hallucinations and a gradual progression toward psychosis with increasing time awake. Frontiers in psychiatry, 9, 303.
- Robinson, R. (2009). Serotonin’s Role in the Pancreas Revealed at Last. PLoS Biol, 7(10), e1000227.
- Greisen, J., Juhl, C. B., Grøfte, T., Vilstrup, H., Jensen, T. S., & Schmitz, O. (2001). Acute pain induces insulin resistance in humans. Anesthesiology: The Journal of the American Society of Anesthesiologists, 95(3), 578-584.
- Li, L., Li, X., Zhou, W., & Messina, J. L. (2013). Acute psychological stress results in the rapid development of insulin resistance. The Journal of endocrinology, 217(2), 175.
- Chou, T. C., Lee, C. E., Lu, J., Elmquist, J. K., Hara, J., Willie, J. T., … & Saper, C. B. (2001). Orexin (hypocretin) neurons contain dynorphin. Journal of Neuroscience, 21(19), RC168-RC168.
- Ramasubbu, R. (2001). Dose–response relationship of selective serotonin reuptake inhibitors treatment‐emergent hypomania in depressive disorders. Acta Psychiatrica Scandinavica, 104(3), 236-239.
- Wurtman, R. J., & Wurtman, J. J. (1995). Brain serotonin, carbohydrate‐craving, obesity and depression. Obesity research, 3(S4), 477S-480S.
- Smith, B. K., York, D. A., & Bray, G. A. (1999). Activation of hypothalamic serotonin receptors reduced intake of dietary fat and protein but not carbohydrate. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 277(3), R802-R811.
- Gregor, M. (2018). How To Reverse Type II Diabetes. NutritionFacts.
- Jornayvaz, F. R., Jurczak, M. J., Lee, H. Y., Birkenfeld, A. L., Frederick, D. W., Zhang, D., … & Shulman, G. I. (2010). A high-fat, ketogenic diet causes hepatic insulin resistance in mice, despite increasing energy expenditure and preventing weight gain. American Journal of Physiology-Endocrinology and Metabolism, 299(5), E808-E815.
- Sun, Z., & Cade, J. R. (1999). A peptide found in schizophrenia and autism causes behavioral changes in rats. Autism, 3(1), 85-95.
- Dohan, F. C. (1988). Genetic hypothesis of idiopathic schizophrenia: its exorphin connection. Schizophrenia bulletin, 14(4), 489-494.
- Zheng, P., Zeng, B., Liu, M., Chen, J., Pan, J., Han, Y., … & Zhou, X. (2019). The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Science advances, 5(2), eaau8317.
- Goto, T., Kubota, Y., Tanaka, Y., Iio, W., Moriya, N., & Toyoda, A. (2014). Subchronic and mild social defeat stress accelerates food intake and body weight gain with polydipsia-like features in mice. Behavioural brain research, 270, 339-348.
- Zhang, J., Fan, Y., Li, Y., Zhu, H., Wang, L., & Zhu, M. Y. (2012). Chronic social defeat up‐regulates expression of the serotonin transporter in rat dorsal raphe nucleus and projection regions in a glucocorticoid‐dependent manner. Journal of neurochemistry, 123(6), 1054-1068.
- Morgan, D., Grant, K. A., Gage, H. D., Mach, R. H., Kaplan, J. R., Prioleau, O., … & Nader, M. A. (2002). Social dominance in monkeys: dopamine D 2 receptors and cocaine self-administration. Nature neuroscience, 5(2), 169-174.
- Sapolsky, R. M. (2005). The influence of social hierarchy on primate health. Science, 308(5722), 648-652.
- Xu, T., Chan, R. C., & Compton, M. T. (2011). Minor physical anomalies in patients with schizophrenia, unaffected first-degree relatives, and healthy controls: a meta-analysis. PloS one, 6(9), e24129.
- Gourion, D., Goldberger, C., Bourdel, M. C., Bayle, F. J., Lôo, H., & Krebs, M. O. (2004). Minor physical anomalies in patients with schizophrenia and their parents: prevalence and pattern of craniofacial abnormalities. Psychiatry research, 125(1), 21-28.
- Oertel-Knöchel, V., & Linden, D. E. (2011). Cerebral asymmetry in schizophrenia. The Neuroscientist, 17(5), 456-467.
- Kay, T., Lehmann, L., & Keller, L. (2019). Kin selection and altruism. Current Biology, 29(11), R438-R442.
- Clarke, F. M., & Faulkes, C. G. (1999). Kin discrimination and female mate choice in the naked mole-rat Heterocephalus glaber. Proceedings of the Royal Society of London. Series B: Biological Sciences, 266(1432), 1995-2002.
- Manica, A., Amos, W., Balloux, F., & Hanihara, T. (2007). The effect of ancient population bottlenecks on human phenotypic variation. Nature, 448(7151), 346-348.
- Yirmiya, K., Segal, N. L., Bloch, G., & Knafo‐Noam, A. (2018). Prosocial and self‐interested intra‐twin pair behavior in monozygotic and dizygotic twins in the early to middle childhood transition. Developmental Science, 21(6), e12665.
- Tornero, E., Sánchez-Romera, J. F., Morosoli, J. J., Vázquez, A., Gómez, Á., & Ordoñana, J. R. (2018). “Altruistic behavior among twins: Willingness to fight and self-sacrifice for their closest relatives”: Correction.
- O’riain, M. J., & Jarvis, J. U. M. (1997). Colony member recognition and xenophobia in the naked mole-rat. Animal Behaviour, 53(3), 487-498.
- Buck, R. (2011). Communicative genes in the evolution of empathy and altruism. Behavior genetics, 41(6), 876-888.
- Ding, Y. C., Chi, H. C., Grady, D. L., Morishima, A., Kidd, J. R., Kidd, K. K., … & Zhang, Y. P. (2002). Evidence of positive selection acting at the human dopamine receptor D4 gene locus. Proceedings of the National Academy of Sciences, 99(1), 309-314.
- Hecht, J. (2008). The Neanderthal correlation. Nature, 453(7194), 562-562.
- Rodrigue, A. L., & Perkins, D. R. (2012). Divergent thinking abilities across the schizophrenic spectrum and other psychological correlates. Creativity Research Journal, 24(2-3), 163-168.
- Abramson, H. A. (1955). Lysergic acid diethylamide (LSD-25): III. As an adjunct to psychotherapy with elimination of fear of homosexuality. The Journal of Psychology, 39(1), 127-155.
- Martin, A. J. (1962). The Treatment of Twelve Male Homosexuals with’LSD'(followed by a Detailed Account of One of them who was a Psychopathic Personality). Acta Psychotherapeutica et Psychosomatica, 394-402.
- Nardou, R., Lewis, E. M., Rothhaas, R., Xu, R., Yang, A., Boyden, E., & Dölen, G. (2019). Oxytocin-dependent reopening of a social reward learning critical period with MDMA. Nature, 569(7754), 116-120.
- Hasselbalch, S. G., Madsen, K., Svarer, C., Pinborg, L. H., Holm, S., Paulson, O. B., … & Knudsen, G. M. (2008). Reduced 5-HT2A receptor binding in patients with mild cognitive impairment. Neurobiology of aging, 29(12), 1830-1838.
I’ve noticed this pattern in my life that whenever I take psychedelics recreationally my depression is lifted for one or two months and I become stress resilent. The first and second time it happened I thought that I was cured “forever”, but I’ve since realized that it always creeps back in. This week I’ve taken LSD for the first time with the primary intent of treating my recent descent into chronic stress and suicidal ideation. It worked better than I expected it to, even though the dose was low (around 40 micrograms). So according to your theory my depression could be related elevated dynorphin. I wonder if doing this consistently could be a sustainable way to manage mood.
LikeLiked by 1 person